The research is primarily focused on understanding different types of protein-lipid interactions which is crucial for designing novel therapeutics related to neurodegenerative disorders (Project 1) and cancer (Project 2). Additionally, through Project 3, the group is also working on potential applicability of a benchtop label-free RI-based technique that can determine free solution binding from conformational and hydration sphere changes, by exploring different protein-ligand bound complexes
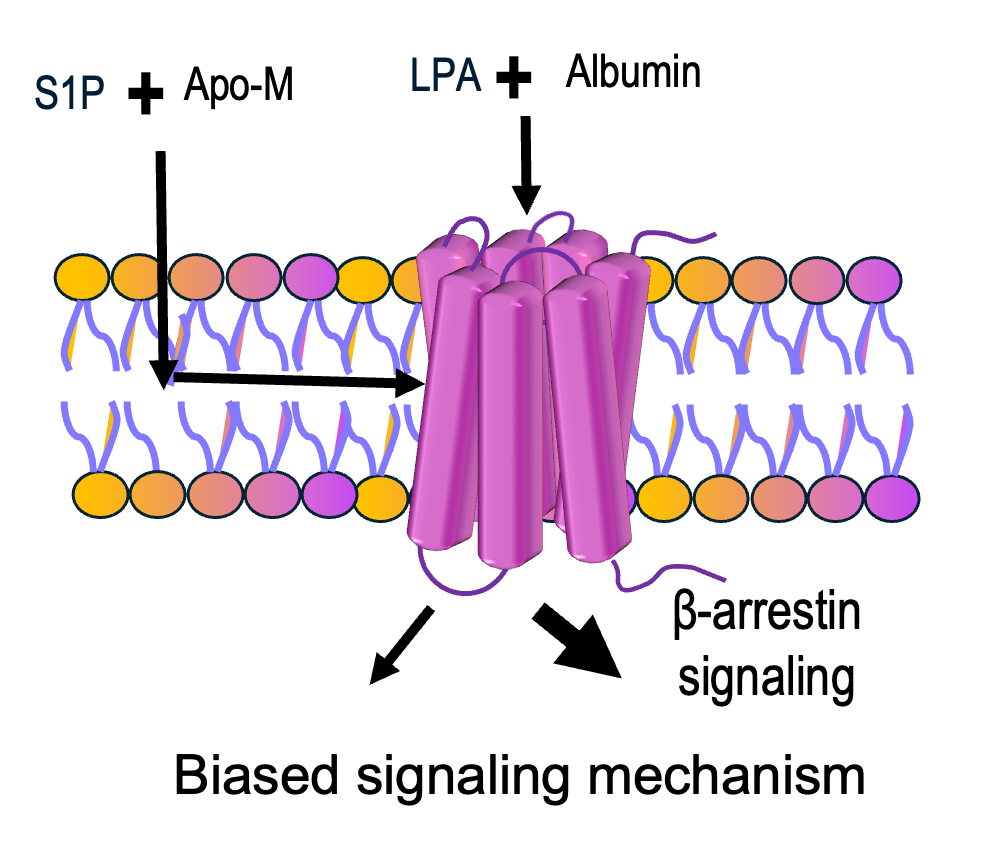
Project 1: Interactions of Bioactive lipids with their chaperons (albumin, HDL) Bioactive lipids signal through G protein-coupled receptor (GPCRs). Lysophosphatidic acid (LPA) and sphingosine-1-phosphate (S1P) are the most common examples of bioactive lipids. LPA activates LPA1 through LPA6 receptors, whereas S1P activates S1P1 through S1P5 receptors. LPA and S1P in plasma are carried by certain lipid chaperones (autotoxin, albumin, apo-M proteins) that have an indirect role to modulate GPCR signaling, known as biased signaling. Biased signaling preferentially activates certain signaling pathways and hence, discovery of biased ligands can potentially fine-tune these GPCR responses in a therapeutic direction. We currently have very limited understanding of this biased signaling mechanism from the lipid chaperones perspective due to unavailable direct probing methods to determine the binding and unbinding of these lipids from their chaperones. The access mechanism of these lysophospholipids by the receptor (through extracellular space or lipid bilayer) is linked towards biasing signaling. Hence we are interested to look at those access mechanism by probing the binding and unbinding of these lipids from their chaperones using fluorescence spectroscopy, label-free technique and computational docking methods.
Project 2: α-lactalbumin-OA complex in HAMLET cis-oleic acid, when complexed with the human milk protein α-lactalbumin (α-La) is widely known as HAMLET (Human Alpha-lactalbumin Made Lethal to Tumor cells). The complex facilitates membrane disintegration upon delivery to the membrane. The formation and dissociation of this protein-lipid complex is elusive due to the misfolding structure of α-La upon oleic acid (OA) binding, and the fate of the protein upon OA transfer to cancer cells remained unknown. These highlight the importance of studying the contributions of lipid binding and transfer mechanisms of this functionally distinct lipid than lysophopholipids to cancer cells. We are interested to investigate the structural and functional properties of such complex to design similar potent protein-lipid complex which can show anticancer activity.
Project 3: Application of the label free technique in probing hydration changes: In this project, we will be looking at the arrangement of hydration sphere around different protein-ligand bound complexes and how would this arrangement can differently respond to RI signal. The project is under construction, and will be updated later.